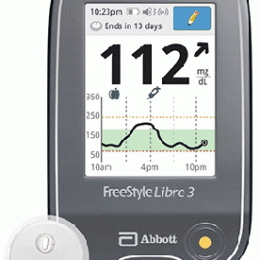
Tag: FreeStyle Libre 3
FDA clears readers for FreeStyle Libre 3
April 14, 2023HME News Staff
ABBOTT PARK, Ill. – Abbott has received clearance from the U.S. Food and Drug Administration (FDA) for a reader for its FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system, which features a small, thin and discreet glucose sensor. With the FDA's clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible. "Our customers all over the world consistently tell us how our FreeStyle...
Abbott receives FDA clearance for FreeStyle Libre 3
June 1, 2022HME News Staff
ABBOTT PARK, Ill. – Abbott has received clearance from the U.S. Food and Drug Administration for its FreeStyle Libre 3 system for people with diabetes age four and up. "The FreeStyle Libre 3 system is a direct result of listening to our customers – and giving them the innovation and sensing technology they've been looking for," said Jared Watkin, senior vice president of Abbott's diabetes care business. "It's a game changer for the millions of people living with diabetes....