F&P’s Evora debuts in US

By HME News Staff
Updated 9:52 AM CDT, Tue April 19, 2022
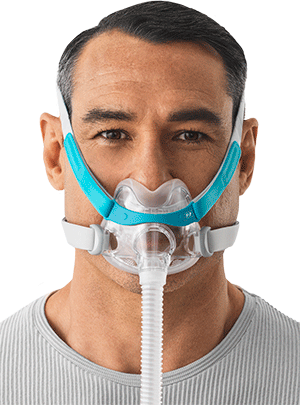 AUCKLAND, New Zealand – Fisher & Paykel Healthcare has launched its Evora Full, a compact full-face mask for obstructive sleep apnea, in the United States. The mask has received FDA 510(k) clearance, paying the way for its sale in the U.S. in early May, following launches in Australia, New Zealand, Europe and Canada. “We welcome this clearance from the FDA and look forward to offering this unique full-face mask to our customers and patients,” said Justin Callahan, president-North America Operations. “The Evora Full marks our entry into the compact full-face mask segment and our teams have worked hard on achieving a final product that optimizes both comfort and performance.” Features of the Evora Full include a floating seal and stability wings to create the next generation of Dynamic Support Technology. F&P says the mask is backed by positive clinical trials showing 91% of participants rating the mask as “stable” or “very stable”; 93% reporting the masks to be “comfortable” or “very comfortable”; and 96% reporting the ability to sleep with the mask in their preferred sleeping position.
AUCKLAND, New Zealand – Fisher & Paykel Healthcare has launched its Evora Full, a compact full-face mask for obstructive sleep apnea, in the United States. The mask has received FDA 510(k) clearance, paying the way for its sale in the U.S. in early May, following launches in Australia, New Zealand, Europe and Canada. “We welcome this clearance from the FDA and look forward to offering this unique full-face mask to our customers and patients,” said Justin Callahan, president-North America Operations. “The Evora Full marks our entry into the compact full-face mask segment and our teams have worked hard on achieving a final product that optimizes both comfort and performance.” Features of the Evora Full include a floating seal and stability wings to create the next generation of Dynamic Support Technology. F&P says the mask is backed by positive clinical trials showing 91% of participants rating the mask as “stable” or “very stable”; 93% reporting the masks to be “comfortable” or “very comfortable”; and 96% reporting the ability to sleep with the mask in their preferred sleeping position.
Comments