FDA clears readers for FreeStyle Libre 3

By HME News Staff
Updated 9:07 AM CDT, Fri April 14, 2023
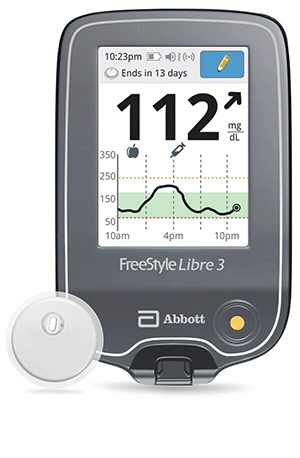 ABBOTT PARK, Ill. – Abbott has received clearance from the U.S. Food and Drug Administration (FDA) for a reader for its FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system, which features a small, thin and discreet glucose sensor. With the FDA's clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible. "Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living," said Jared Watkin, senior vice president for Abbott's diabetes care business. "The FreeStyle 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems." The FreeStyle Libre 3 reader is a small handheld device that displays real-time glucose readings directly from a small sensor worn on the back of a person's upper arm, allowing them to manage their diabetes quickly and easily by viewing their glucose readings6 on a large, bright and easy-to-see screen. People who use the FreeStyle Libre 3 system will still have the option to use the current FreeStyle Libre 3 smartphone apps.
ABBOTT PARK, Ill. – Abbott has received clearance from the U.S. Food and Drug Administration (FDA) for a reader for its FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system, which features a small, thin and discreet glucose sensor. With the FDA's clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible. "Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living," said Jared Watkin, senior vice president for Abbott's diabetes care business. "The FreeStyle 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems." The FreeStyle Libre 3 reader is a small handheld device that displays real-time glucose readings directly from a small sensor worn on the back of a person's upper arm, allowing them to manage their diabetes quickly and easily by viewing their glucose readings6 on a large, bright and easy-to-see screen. People who use the FreeStyle Libre 3 system will still have the option to use the current FreeStyle Libre 3 smartphone apps.
Comments